Product Overview
UPC #: 353731002066
SKU #: 000000000300005140
Take one capsule twice daily with or without food, or as directed by your physician.
Store at 20° to 25°C (68° to 77°F).
For use under supervision of a physician. Keep this and all medications out of the reach of children.
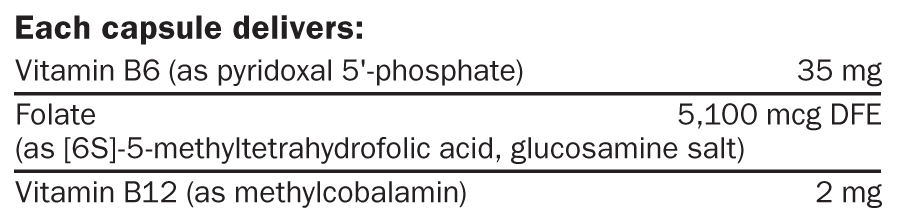
Alpha-lipoic acid, capsule (hypromellose, sodium copper chlorophyllin, and water), pyridoxal 5’-phosphate, vegetable stearic acid, vegetable magnesium stearate, silica, (6S)-5-methyltetrahydrofolic acid glucosamine salt,S1 microcrystalline cellulose, and methylcobalamin.
S1. Quatrefolic® is a registered trademark of Gnosis S.p.A. Produced under U.S. patent 7,947,662.

 Soy
Soy  Egg
Egg  Gluten
Gluten  Shellfish
Shellfish  Animal Products
Animal Products  Dairy
Dairy